19.Elaborate the reactions of the electron transport chain with a note on the electrochemical gradient.
19.Elaborate the reactions of the electron transport chain with a note on the electrochemical gradient.
answer:
Electron Transport Chain: An Introduction
The electron transport chain is a protein cluster that transfers electrons through a membrane within mitochondria to form a proton gradient that drives the production of adenosine triphosphate (ATP). The cell uses ATP as an energy source for metabolic processes and cellular functions.
In mammalian cells, the electron transport chain (ETC) is the primary O2 consumer. The ETC is responsible for transporting electrons from NADH and FADH2 to protein complexes and mobile electron carriers. In the ETC, Coenzyme Q (CoQ) and Cyt c are mobile electron carriers, and O2 is the final electron recipient.
What is the Electron Transport Chain?
The electron transport system occurs in the mitochondrial cristae, which contains a number of cytochromes and coenzymes.
These cytochromes and coenzymes act as transporter atoms, moving particles around.
They accept high-energy electrons and transfer them to the next atom in the system.
The energy of electrons transports protons across the layer into the mitochondrion's outer membrane at key proton-pumping sites.
Each NADH particle is extremely energetic, representing the exchange of six protons into the mitochondrion's outer compartment.
Each FADH2 atom represents a four-proton exchange.
The electron progression is similar to that of photosynthesis. Electrons move from NAD to FAD, then to various cytochromes and coenzymes, eventually losing their electrons.
The final electron acceptor in cellular respiration is an oxygen molecule. The electrons combine with an oxygen molecule in their energy-depleted state. The electron-oxygen reaction then produces two hydrogen particles (protons) to form a water particle (H2O).
Functions of the Electron Transport Chain
It serves two important purposes:
Regeneration of Electron Carriers: NADH and FADH2 transfer electrons to the electron transport chain, reverting to NAD+ and FAD. This is significant because the oxidised forms of these electron carriers are required for glycolysis and the citric acid cycle to function properly.
Generates a Proton Gradient: The transport chain creates a proton gradient across the inner mitochondrial membrane, with more H+ in the intermembrane space and less in the matrix. This gradient represents a form of stored energy and is used to make ATP.
Location of Electron Transport Chain
As the citric acid cycle occurs in the mitochondria, high-energy electrons are also present within the mitochondria. As a result, in eukaryotes, the electron transport chain also occurs in the mitochondria.
Mitochondria are organelles inside eukaryotic cells that produce adenosine triphosphate (ATP), the fundamental energy atom utilised by the cell.
The outer membrane of mitochondria is permeable to different particles since it contains a mitochondrial porin, which is a pore-shaping protein.
The inner membrane of mitochondria is impermeable to most ions and polar atoms and only permeable to ATP, pyruvate and citrate due to metabolite carriers.
Electron Transport Chain Complexes
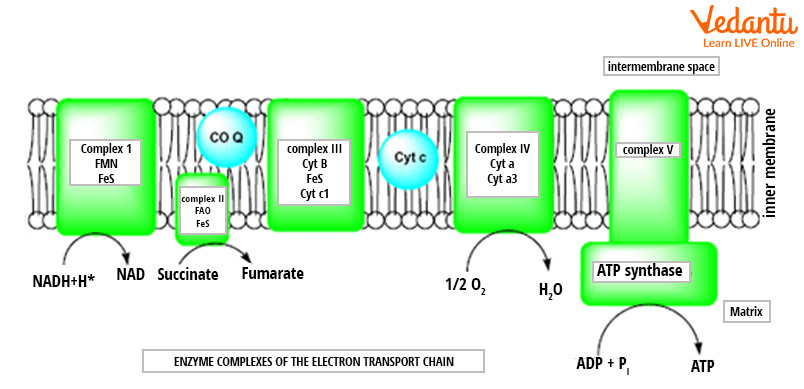
Image: Electron Transport Chain Diagram
The electron transport chain has four complexes:
Complex 1-NADH-Q Oxidoreductase:
It includes enzymes comprising iron-sulphur and FMN.
Here, two electrons are carried to the first complex aboard NADH.
FMN is derived from vitamin B2.
Complex 2-Succinate-Q Reductase:
FADH2 that isn't gone through complex 1 is received straightforwardly from complex 2.
Compound ubiquinone (Q) combines first and second complexes.
The Q atom is dissolvable in water and moves uninhibitedly in the hydrophobic core of the membrane.
In this stage, an electron is conveyed straightforwardly to the electron protein chain.
The quantity of ATP obtained at this stage is straightforwardly relative to the number of protons that are pumped across the inner layer of the mitochondria.
Complex 3-Cytochrome C Reductase:
This complex includes Fe-S protein, Cytochrome b, and Cytochrome c proteins.
Cytochrome proteins comprise the heme group.
This complex is liable for pumping protons across the membrane.
It likewise passes electrons to the cytochrome c where it is moved to the fourth complex of enzymes and proteins.
Here, Q is the electron giver and Cytochrome C is the electron acceptor.
Complex 4-Cytochrome c Oxidase:
This complex involves cytochrome c, a and a3.
There are two heme groups and every one of them is available in cytochrome c and a3.
The cytochromes are answerable for holding oxygen atoms among copper and iron until the oxygen content is totally diminished.
In this stage, the decreased oxygen picks two hydrogen particles from the surroundings to make water.
Inhibitors of Electron Transport Chain
Conclusion
The final and most important step in cellular respiration is the electron transport chain. Glycolysis and the citric acid cycle are used to produce the necessary precursors, whereas the electron transport chain produces the vast majority of ATP. It is essential for both photosynthesis and cellular respiration. This article gives insight into the location, function and major components of the electron transport chain. The final acceptor electron in the transport chain is oxygen.
Comments
Post a Comment