Peptide bonds are unusually stable when compared to other linkages. Justify with a neat diagram.
Peptide bonds are unusually stable when compared to other linkages. Justify with a neat diagram.
answer:
Why peptide bond is stronger
1. Peptide bonds are strong with partial double bond character:
- They are not broken by heating or high salt concentration.
- They can be broken by exposing them to strong acid or base for a long time at elevated temperature. Also by some specific enzymes (digestive enzymes).
2. Peptide bonds are rigid and planar bonds therefore they stabilize protein structure.
3. Peptide bond contains partial positive charge groups (polar hydrogen atoms of amino groups) and partial negative charge groups (polar oxygen atoms of carboxyl groups).
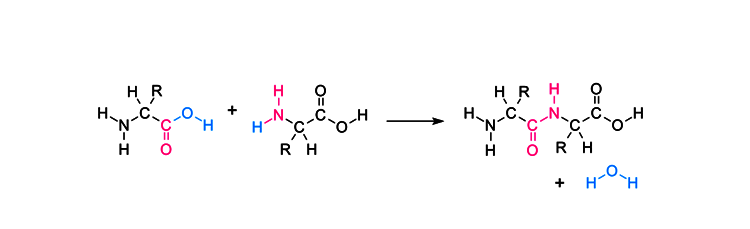
Peptide Bond Formation or Synthesis
A peptide bond is formed by a dehydration synthesis or reaction at a molecular level. This reaction is also known as a condensation reaction which usually occurs between amino acids.
As depicted in the figure given below, two amino acids bond together to form a peptide bond by the dehydration synthesis. During the reaction, one of the amino acids gives a carboxyl group to the reaction and loses a hydroxyl group (hydrogen and oxygen).

The other amino acid loses hydrogen from the NH2 group. The hydroxyl group is substituted by nitrogen thus forming a peptide bond. This is one of the primary reasons for peptide bonds being referred to as substituted amide linkages. Both the amino acids are covalently bonded to each other.
The newly formed amino acids are also called a dipeptide.
Let’s have a look at a simpler diagram depicting the formation of the peptide bond.
During the reactions that occur, the resulting CO-NH bond is the peptide bond, and the resulting molecule is an amide. The four-atom functional group -C(=O)NH- is called an amide group or a peptide group.
Comments
Post a Comment